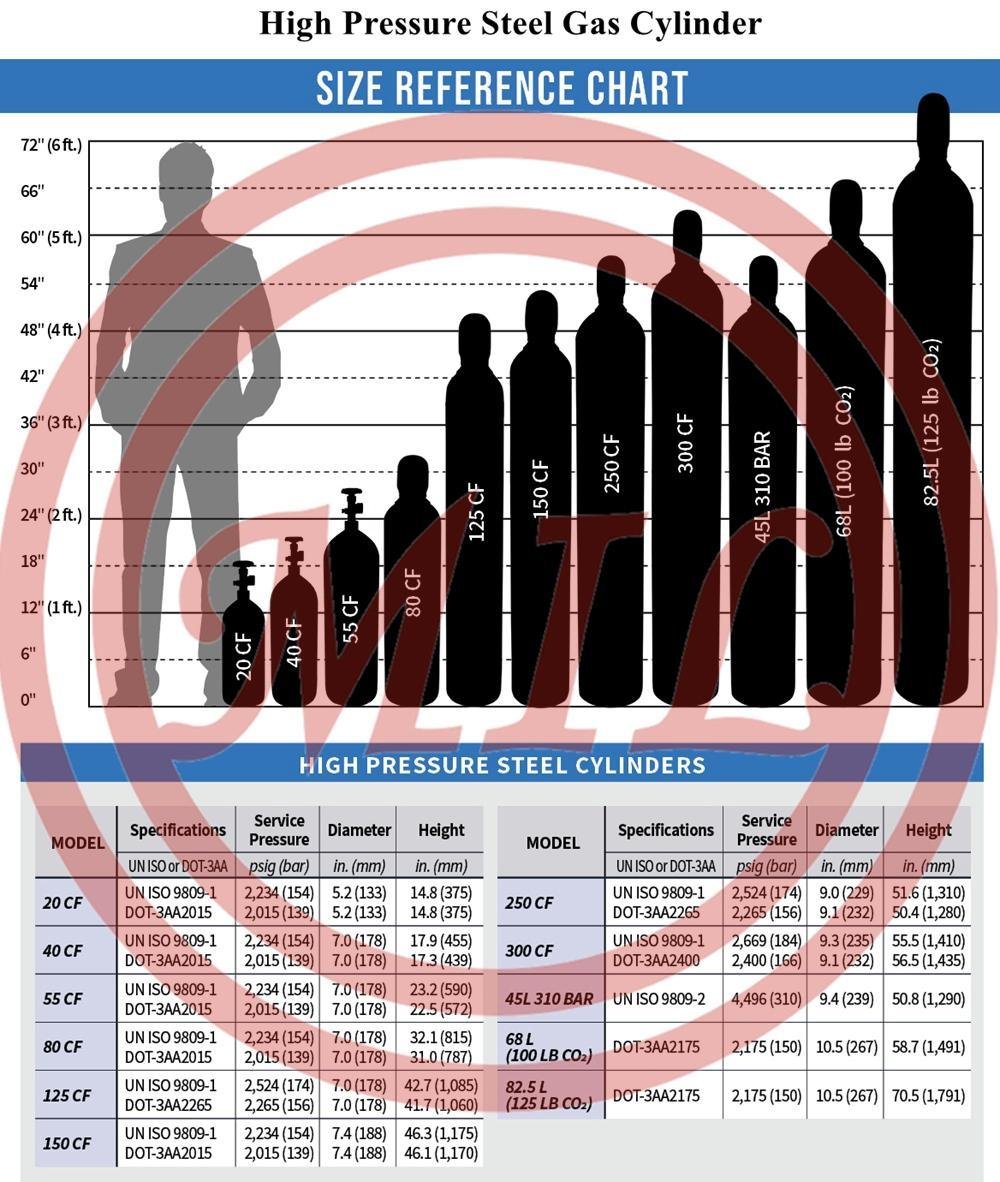
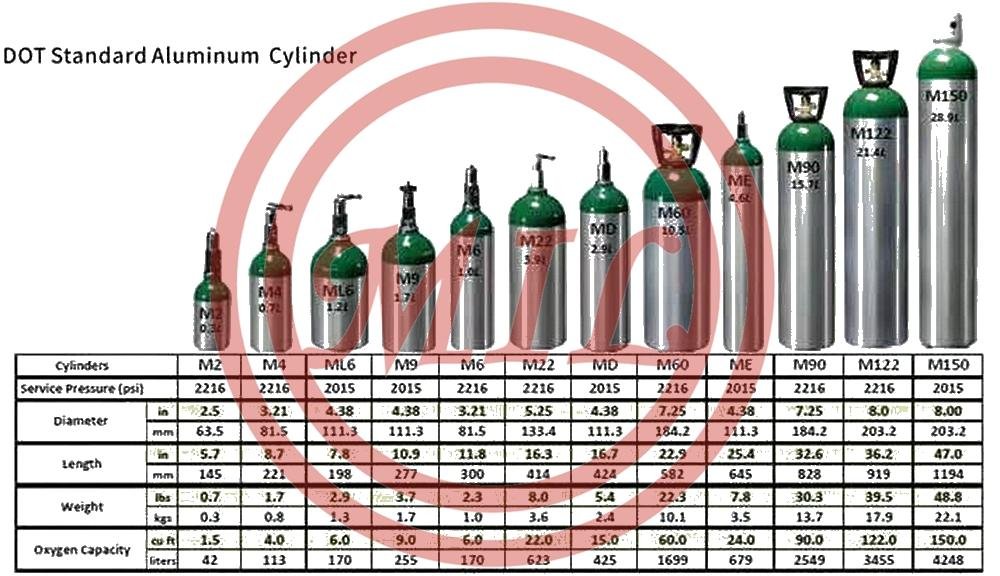
Product Description
Gas Cylinder
A gas cylinder is a pressure vessel able to contain and store gases at pressures much higher than atmospheric levels. High pressure cylinders can also be referred to as tanks or bottles. Within the container, the contents can be in a state of compressed gas, vapor over liquid, supercritical fluid, or dissolved in a substrate material.
In order to release the gas held inside in a controllable manner, these cylinders are typically fitted with a valve and nozzle at the top. This allows the cylinder to be connected to the appropriate reception apparatus. Most people are familiar with the oxygen tanks used for scuba diving or the red fire extinguishers commonly found in workplaces and apartment buildings; both of these are examples of gas cylinders.
These cylinders can be made from all kinds of materials, including carbon steel, but most modern high-pressure cylinders are made from aluminum, stainless steel, or carbon fiber; the latter materials all having a combination of strength, light weight and corrosion resistance.
They can also be found in all kinds of industries. Any time it is necessary to safely transport and store compressed gases, high pressure cylinders are the solution. Their availability in a variety of shapes, sizes and weights means they are adaptable to many situations. One factor that goes into the adaptability of these tanks is the choice of material. With so many different stainless steel alloys at their disposal, manufacturers are able to tailor the tank to the desired specifications.
The types of gaseous substances that are regularly stored in high pressure cylinders include oxygen, helium, silicon hydrides, hydrogen, krypton, nitrogen, argon and fluorine. There is another class of gases that liquefy when placed under high pressure, such as carbon dioxide, propane, sulfur dioxide, nitrous oxide, butane and ammonia.
Furthermore, some cylinders are used to hold substances that liquefy under high pressure and lowered temperatures, such as liquid nitrogen, liquid hydrogen and liquid oxygen. These are known as cryogenic gases. In order to house them safely, the cylinders will need to be equipped with a bleed device that will prevent overpressure from rupturing the container.
Finally, there’s what’s known as dissolved gases. These tend to be very unstable and can explode even at atmospheric pressure. In order to prevent this, the cylinders are filled with a porous, inert material that the gas will be saturated into in order to make the solution stable. The primary example of this is acetylene (it’s the same principle under which carbon dioxide is infused into a soft drink).
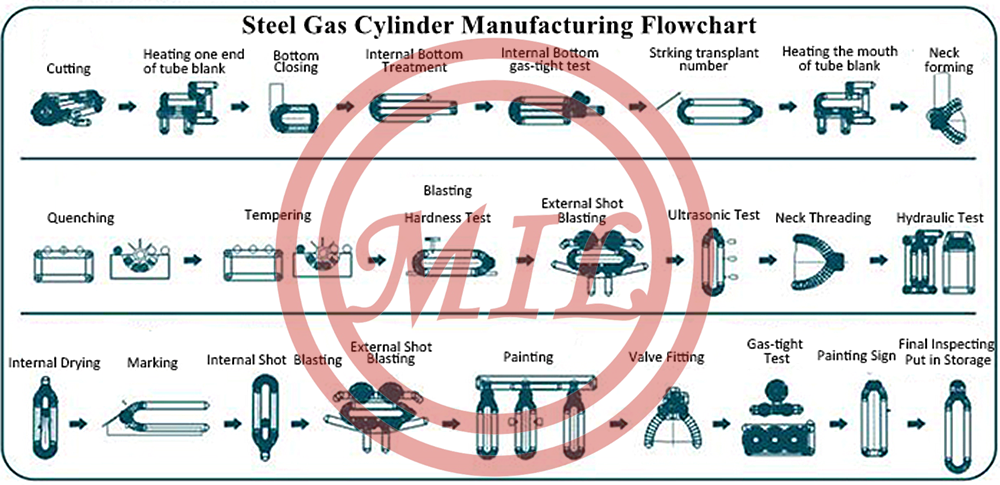
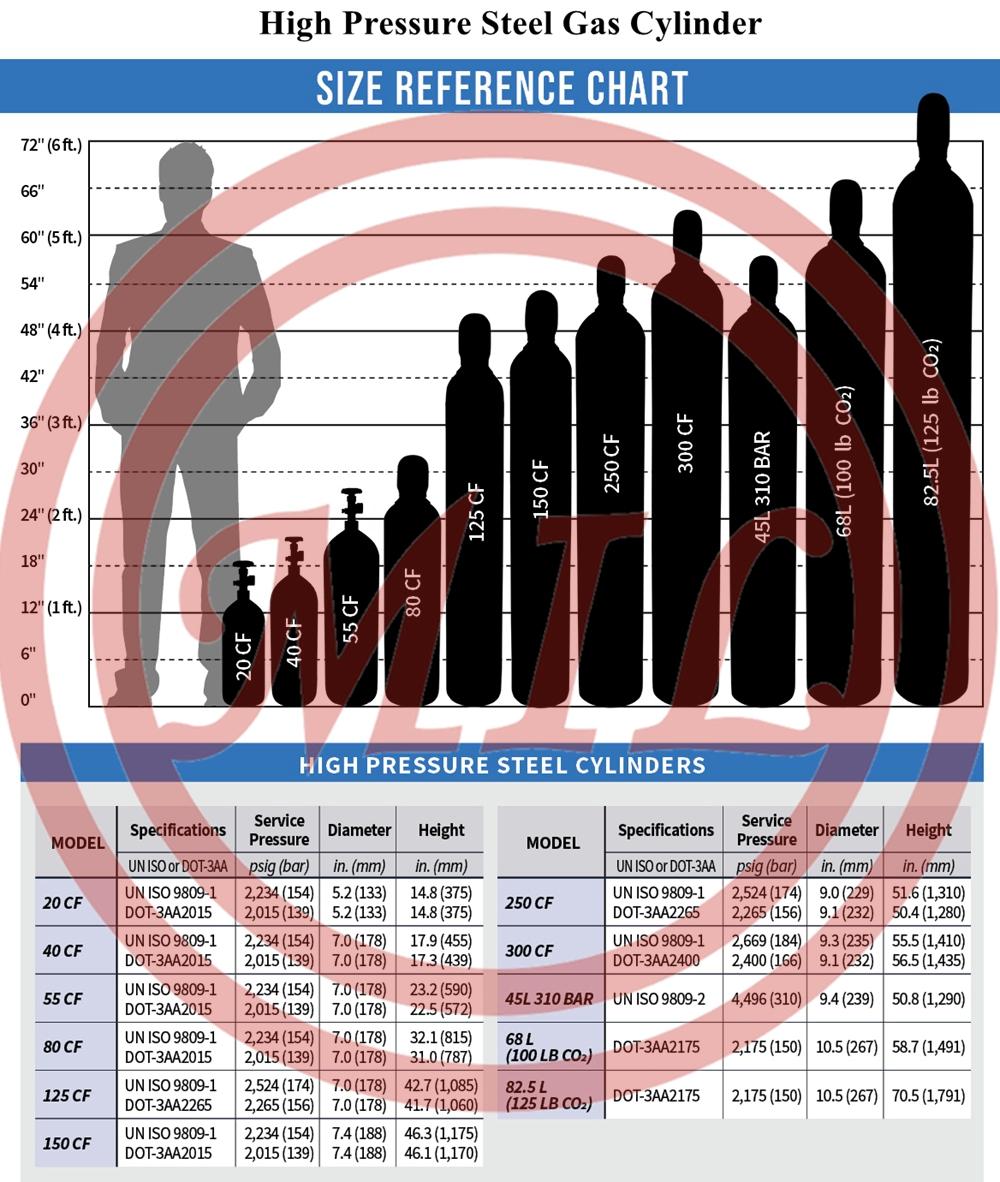
Product Image